Spatial co-expression#
- Date:
4/10/23
1 Dataset explanation#
Here we will use the visium mini dataset, which is a subset of the Visium 10X mouse brain dataset, to illustrate how to perform a spatial co-expression analysis. This mini visium dataset has been fully processed and is easily available through the GiottoData package.
2 Start Giotto#
# Ensure Giotto Suite is installed
if(!"Giotto" %in% installed.packages()) {
devtools::install_github("drieslab/Giotto@suite")
}
library(Giotto)
# Ensure Giotto Data is installed
if(!"GiottoData" %in% installed.packages()) {
devtools::install_github("drieslab/GiottoData")
}
library(GiottoData)
# Ensure the Python environment for Giotto has been installed
genv_exists = checkGiottoEnvironment()
if(!genv_exists){
# The following command need only be run once to install the Giotto environment
installGiottoEnvironment()
}
3 Load the mini Giotto object#
visium = loadGiottoMini(dataset = 'visium')
1. read Giotto object
2. read Giotto feature information
3. read Giotto spatial information
4. read Giotto image information
no external python path was provided, but a giotto python environment was found
and will be used
no external python path was provided, but a giotto python environment was found
and will be used
4 Calculate spatially variable genes#
make sure that you have already created a spatial network
you can identify spatial genes with any type of method, here we will use the binSpect method
showGiottoSpatNetworks(visium)
└──Spatial unit "cell"
├──S4 spatialNetworkObj "Delaunay_network" (1770 rows)
│ from to sdimx_begin sdimy_begin sdimx_end
│ 1: AAAGGGATGTAGCAAG-1 TCAAACAACCGCGTCG-1 5477 -4125 5340
│ 2: AAAGGGATGTAGCAAG-1 ACGATCATACATAGAG-1 5477 -4125 5546
│ 3: AAAGGGATGTAGCAAG-1 TATGCTCCCTACTTAC-1 5477 -4125 5408
│ 4: AAAGGGATGTAGCAAG-1 TTGTTCAGTGTGCTAC-1 5477 -4125 5615
│ sdimy_end distance weight
│ 1: -4125 137.0000 0.007299270
│ 2: -4244 137.5573 0.007269700
│ 3: -4244 137.5573 0.007269700
│ 4: -4125 138.0000 0.007246377
│
└──S4 spatialNetworkObj "spatial_network" (3288 rows)
from to sdimx_begin sdimy_begin sdimx_end
1: AAAGGGATGTAGCAAG-1 TCAAACAACCGCGTCG-1 5477 -4125 5340
2: AAAGGGATGTAGCAAG-1 ACGATCATACATAGAG-1 5477 -4125 5546
3: AAAGGGATGTAGCAAG-1 TATGCTCCCTACTTAC-1 5477 -4125 5408
4: AAAGGGATGTAGCAAG-1 TTGTTCAGTGTGCTAC-1 5477 -4125 5615
sdimy_end distance weight
1: -4125 137.0000 0.007246377
2: -4244 137.5573 0.007217233
3: -4244 137.5573 0.007217233
4: -4125 138.0000 0.007194245
ranktest = binSpect(visium, bin_method = 'rank',
calc_hub = T, hub_min_int = 5,
spatial_network_name = 'Delaunay_network')
This is the single parameter version of binSpect
1. matrix binarization complete
2. spatial enrichment test completed
3. (optional) average expression of high expressing cells calculated
4. (optional) number of high expressing cells calculated
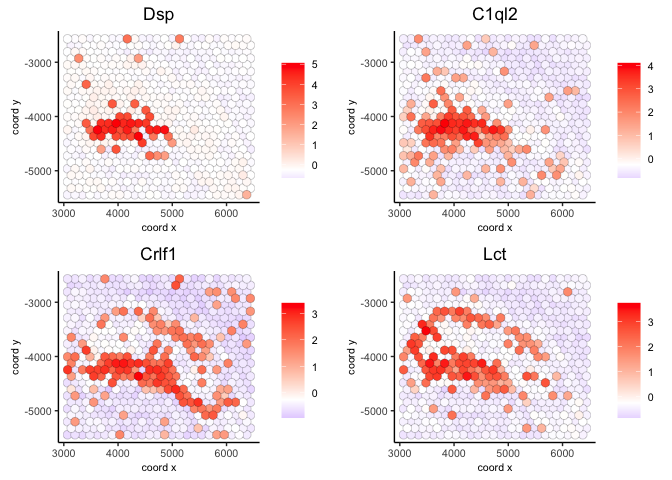
spatFeatPlot2D(visium,
expression_values = 'scaled',
feats = ranktest$feats[1:6], cow_n_col = 2, point_size = 1.5)
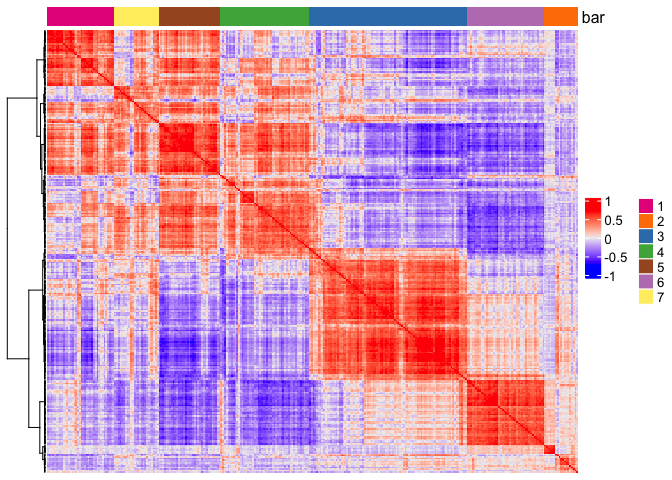
7 Create metagenes/co-expression modules and visualize#
extract a balanced set of genes from each co-expression module.
aggregate genes into metagenes.
# 3.4 create metagenes / co-expression modules
cluster_genes = getBalancedSpatCoexpressionFeats(spat_cor_netw_DT, maximum = 30)
[1] 1
[1] 2
There are only 19 features for cluster 2
Maximum will be set to 19
[1] 3
[1] 4
[1] 5
[1] 6
[1] 7
There are only 25 features for cluster 7
Maximum will be set to 25
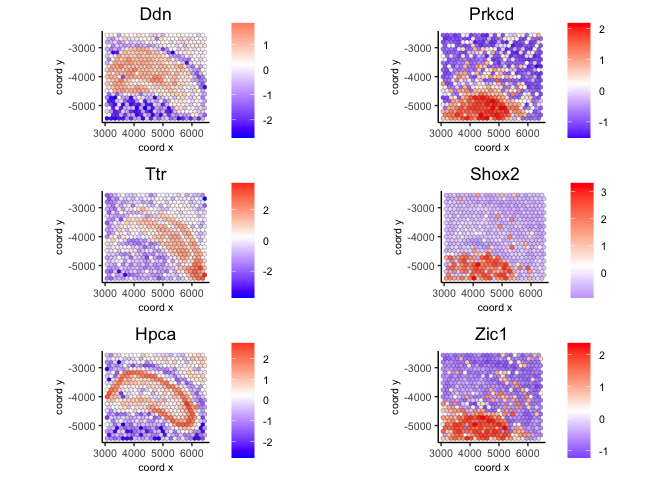
visium = createMetafeats(visium, feat_clusters = cluster_genes, name = 'cluster_metagene')
cluster_metagene has already been used, will be overwritten
> " cluster_metagene " already exists and will be replaced with new spatial
enrichment results
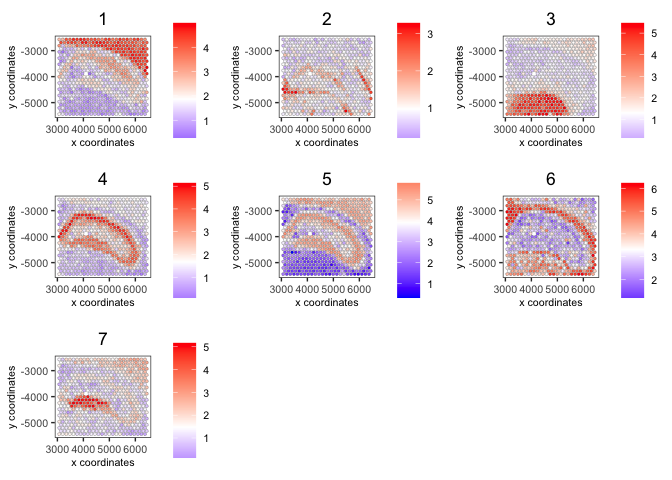
spatCellPlot(visium,
spat_enr_names = 'cluster_metagene',
cell_annotation_values = as.character(c(1:7)),
point_size = 1, cow_n_col = 3)
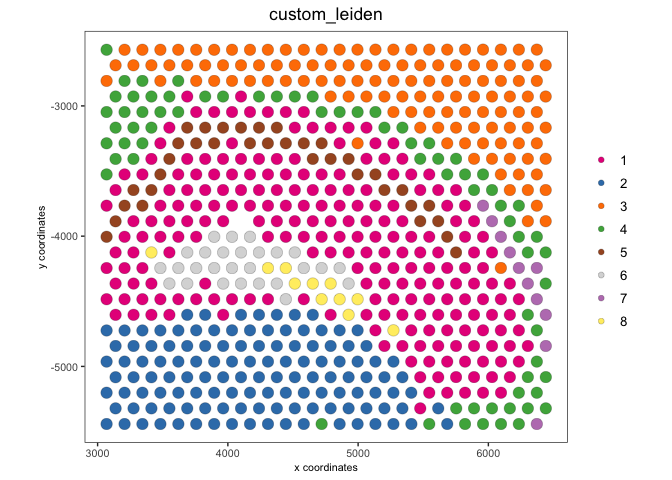
8 (optional) Spatially informed clustering#
Here we illustrate how to use information from #5 as input for clustering using the standard pipeline (PCA > nearest-neighbors > leiden clusters).
my_spatial_genes = names(cluster_genes)
visium <- runPCA(gobject = visium,
feats_to_use = my_spatial_genes,
name = 'custom_pca')
a custom vector of genes will be used to subset the matrix
class of selected matrix: dgCMatrix
Warning in (function (A, nv = 5, nu = nv, maxit = 1000, work = nv + 7, reorth =
TRUE, : You're computing too large a percentage of total singular values, use a
standard svd instead.
custom_pca has already been used, will be overwritten
> custom_pca already exists and will be replaced with new dimension reduction
object
visium <- runUMAP(visium,
dim_reduction_name = 'custom_pca',
dimensions_to_use = 1:20,
name = 'custom_umap')
custom_umap has already been used, will be overwritten
> custom_umap already exists and will be replaced with new dimension reduction
object
visium <- createNearestNetwork(gobject = visium,
dim_reduction_name = 'custom_pca',
dimensions_to_use = 1:20, k = 5,
name = 'custom_NN')
IGRAPH dbea2e8 DNW- 624 1872 --
+ attr: name (v/c), weight (e/n), distance (e/n), shared (e/n), rank
| (e/n)
+ edges from dbea2e8 (vertex names):
[1] AAAGGGATGTAGCAAG-1->AACTGGGTCCCGACGT-1
[2] AAAGGGATGTAGCAAG-1->CAGCTCGTGCTTGTGT-1
[3] AAAGGGATGTAGCAAG-1->CGTACCTGATAGGCCT-1
[4] AAATGGCATGTCTTGT-1->GTGCACGAAAGTGACT-1
[5] AAATGGCATGTCTTGT-1->CTCTGCGAAGCAAGCA-1
[6] AAATGGCATGTCTTGT-1->GATATCTCATGCAATA-1
[7] AAATGGTCAATGTGCC-1->CGAAGTTGCTCTGTGT-1
+ ... omitted several edges
> " custom_NN " already exists and will be replaced with new nearest neighbor
network
visium <- doLeidenCluster(gobject = visium,
network_name = 'custom_NN',
resolution = 0.15, n_iterations = 1000,
name = 'custom_leiden')
custom_leiden has already been used, will be overwritten
spatPlot2D(visium,
cell_color = 'custom_leiden', point_size = 4)
9 Session Info#
sessionInfo()
R version 4.2.2 (2022-10-31)
Platform: aarch64-apple-darwin20 (64-bit)
Running under: macOS Ventura 13.2.1
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.2-arm64/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.2-arm64/Resources/lib/libRlapack.dylib
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] GiottoData_0.2.1 Giotto_3.2.1
loaded via a namespace (and not attached):
[1] rsvd_1.0.5 Rcpp_1.0.10 here_1.0.1
[4] lattice_0.20-45 circlize_0.4.15 FNN_1.1.3.1
[7] png_0.1-8 rprojroot_2.0.3 digest_0.6.31
[10] foreach_1.5.2 utf8_1.2.3 R6_2.5.1
[13] stats4_4.2.2 evaluate_0.20 ggplot2_3.4.1
[16] pillar_1.8.1 GlobalOptions_0.1.2 zlibbioc_1.44.0
[19] rlang_1.0.6 irlba_2.3.5.1 rstudioapi_0.14
[22] data.table_1.14.8 magick_2.7.3 S4Vectors_0.36.1
[25] GetoptLong_1.0.5 Matrix_1.5-3 reticulate_1.28
[28] rmarkdown_2.20 labeling_0.4.2 BiocParallel_1.32.5
[31] uwot_0.1.14 beachmat_2.14.0 igraph_1.4.0
[34] munsell_0.5.0 DelayedArray_0.24.0 BiocSingular_1.14.0
[37] compiler_4.2.2 xfun_0.37 pkgconfig_2.0.3
[40] BiocGenerics_0.44.0 shape_1.4.6 htmltools_0.5.4
[43] tidyselect_1.2.0 tibble_3.1.8 IRanges_2.32.0
[46] codetools_0.2-19 matrixStats_0.63.0 fansi_1.0.4
[49] crayon_1.5.2 dplyr_1.1.0 withr_2.5.0
[52] grid_4.2.2 jsonlite_1.8.4 gtable_0.3.1
[55] lifecycle_1.0.3 magrittr_2.0.3 scales_1.2.1
[58] ScaledMatrix_1.6.0 cli_3.6.0 dbscan_1.1-11
[61] farver_2.1.1 XVector_0.38.0 doParallel_1.0.17
[64] generics_0.1.3 vctrs_0.5.2 cowplot_1.1.1
[67] rjson_0.2.21 RColorBrewer_1.1-3 iterators_1.0.14
[70] tools_4.2.2 Biobase_2.58.0 glue_1.6.2
[73] MatrixGenerics_1.10.0 parallel_4.2.2 fastmap_1.1.0
[76] yaml_2.3.7 clue_0.3-64 colorspace_2.1-0
[79] terra_1.7-18 cluster_2.1.4 ComplexHeatmap_2.14.0
[82] knitr_1.42